Introduction
The patentability of plants has been a long running legal and political saga at the EPO. Following an intervention this month by the president of the EPO, it appears uncertainty in this area is set to continue for the foreseeable future.
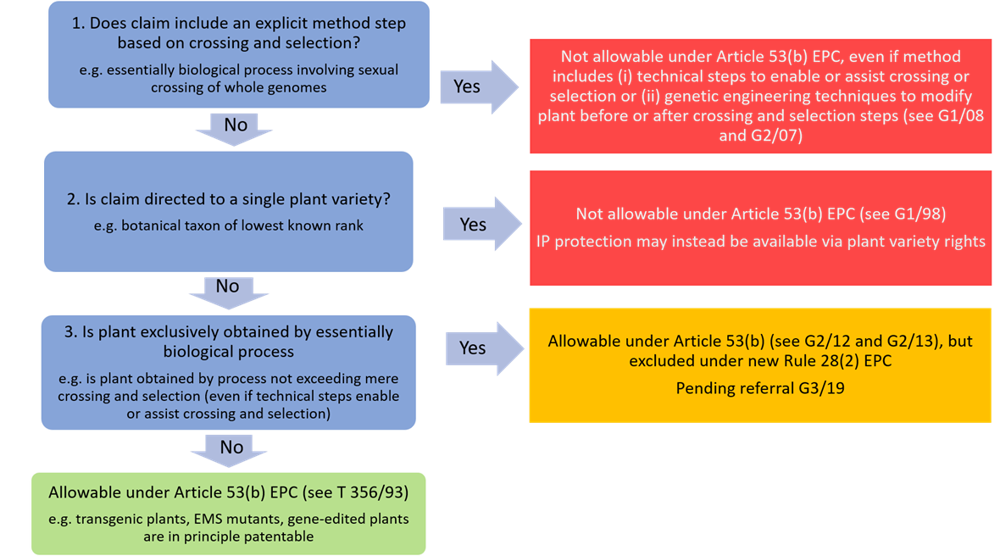
An overview of the EPO’s current position regarding the patenting of plant is depicted in Figure 1.
- Method claims that explicitly recite any step of sexual crossing of the whole genome are not allowable under Article 53(b) EPC. This exclusion from patentability applies even if the method claim includes any technical steps to enable or assist crossing and selection (e.g. marker assisted breeding). This exclusion from patentability also applies if the method claim includes any genetic engineering techniques to modify the plant before or after crossing and selection (e.g. purposeful insertion or modification of genes).
- Plant claims to a single variety (e.g. botanical taxon of lowest known rank) are not allowable under Article 53(b) EPC. However, claims drafted more broadly to species, genus or higher are in principle patentable.
- Plants produced by genetic engineering techniques are allowable under Article 53(b) EPC. For example, transgenic, EMS mutant or genome-edited plants are allowable under Article 53(b) EPC. However, uncertainty still remains over the patentability of plants obtained merely by crossing and selection (even if technical steps such as marker assisted breeding were used to select the plants).
Figure 1. The patentability of plants at the EPO
Contentious issues
The point of contention is still whether or not plants exclusively obtained by essentially biological processes are themselves patentable. It appeared this issue was resolved in 2015 with the Enlarged Board of Appeal of the EPO ruling in G2/12 and G2/13 [1] (the so-called "Tomato and Broccoli II" decisions) that plants exclusively obtained by means of an essentially biological process are allowable under Article 53(b) EPC.
As previously reported [2], however, the European Commission is concerned this interpretation of Article 53(b) EPC is in conflict with the legal protection provided to plant varieties under Community Plant Variety Rights. Following an intervention by the European Commission, the Administrative Council of the EPO introduced Rule 28(2) EPC in 2017 that states:
“Under Article 53(b) EPC, European Patents shall not be granted in respect of plants or animals exclusively obtained by means of an essentially biological process. [emphasis added]”
Rule 28(2) EPC is therefore intentionally in direct conflict with the interpretation of Article 53(b) EPC reached by the Enlarged Board of Appeal in Decisions G2/12 and G2/13. This has raised fresh uncertainty over whether or not plants exclusively obtained by essentially biological processes are themselves patentable.
Under Article 164(2) EPC, the provisions of the European Patent Convention (e.g. “Articles”) should prevail over the Implementing Regulations (e.g. “Rules”). The EPO Board of Appeal therefore found in decision T 1063/18 (the so-called "Pepper" decision) [3] that Article 53(b) EPC (as interpreted in G2/12 and G2/13) prevails over new Rule 28(2) EPC. Thus, the Board of Appeal found that plants exclusively obtained by essentially biological processes are in principle patentable at the EPO.
Pending referral G3/19
The EPO’s committee on patent law including representatives of the 38 EPC Contracting States (and observers from the European Commission) have supported measures to obtain an opinion from the Enlarged Board of Appeal following the decision T1063/18. Despite some controversy and question marks being raised over the legal validity of this approach, the EPO President has this month referred the following points of law to the Enlarged Board of Appeal:
- Having regard to Article 164(2) EPC, can the meaning and scope of Article 53 EPC be clarified in the Implementing Regulations to the EPC without this clarification being a priori limited by the interpretation of said Article given in an earlier decision of the Boards of Appeal or the Enlarged Board of Appeal?
- If the answer to question 1 is yes, is the exclusion from patentability of plants and animals exclusively obtained by means of an essentially biological process pursuant to Rule 28(2) EPC in conformity with Article 53(b) EPC which neither explicitly excludes nor explicitly allows said subject-matter?
This referral is currently pending as G3/19 [4]. By referring these points of law to the Enlarged Board of Appeal, the EPO president is seeking for the Enlarged Board of Appeal to further consider the ruling previously made in the G2/12 and G2/13 decisions.
Next Steps
Under Article 112(1)(b) EPC, the president of the EPO may only refer a point of law to the Enlarged Board of Appeal where two Boards of Appeal have given different decisions on that question. It is therefore possible that the referral to the Enlarged Board of Appeal will not be admitted. Even if admitted, the Enlarged Board of Appeal may simply re-iterate their previous finding that plants exclusively obtained by means of an essentially biological process are allowable under Article 53(b) EPC. In this scenario, a change in law may instead require the more complex route of a Court of Justice of the EU (CJEU) ruling on the interpretation of article 4(1)(b) of the Biotech Directive, followed by Amendment of Article 53(b) EPC itself to bring into line with EU law [5].
The current debate has underlined the need for legal certainty in the interest of users of the European patent system and general public. However, it appears that uncertainty over the patentability of plants is set to continue for the foreseeable future. The unfolding events may also have broader implications on the institutional independence of the EPO’s Boards of Appeal.
We will provide further commentary once the implications of the EPO President's intervention are clearer. If you would like any further information, please contact Huw Jenkins (hjenkins@secerna.com).
2. https://secerna.co.uk/news/update-on-the-patentability-of-plants
3. https://www.epo.org/law-practice/case-law-appeals/recent/t181063eu1.html
4. https://www.epo.org/law-practice/case-law-appeals/eba/pending.html
5. http://www.cipa.org.uk/policy-and-news/briefing-papers/patenting-of-plants-in-europe-position-paper/