Welcome to “A to Z: Drugs in Highlight
This series is designed to delve into the intricate realm of pharmaceutical drugs. As the world of pharmaceuticals continues to evolve with new discoveries and emerging challenges, we aim to shed light on the diverse array of medications available today, ranging from blockbuster medications to those that may not be as familiar. Whether you’re a healthcare professional, a student, or simply someone with a curious mind, “A to Z: Drugs in Highlight” promises to be an enlightening and engaging series.
U is for UPTRAVI®
UPTRAVI® (from Johnson & Johnson) is used to treat pulmonary arterial hypertension (PAH) in adults.
How it works:
PAH is a type of pulmonary hypertension where the blood vessels in the lungs become narrowed. This narrowing increases pressure in the pulmonary arteries, which are responsible for carrying blood from the heart to the lungs. As a result, the heart struggles to pump blood efficiently through the lungs. PAH symptoms often develop gradually and may go unnoticed until the condition advances. Common symptoms include shortness of breath, chest pain or pressure, fatigue, dizziness or fainting, swelling in the ankles, legs and abdomen.
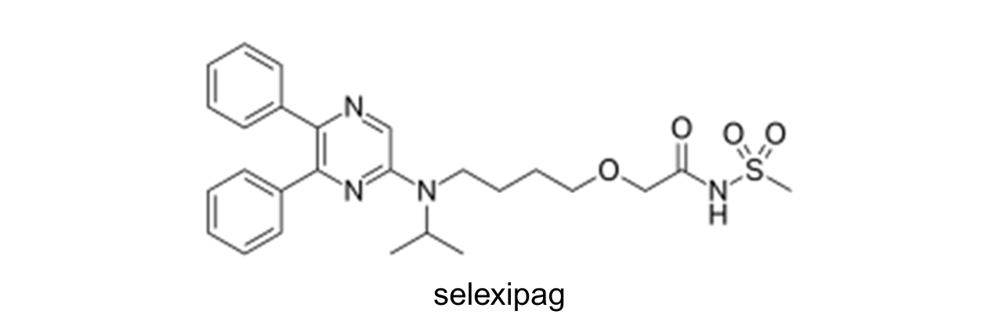
UPTRAVI® is prescribed for adults with functional class II PAH (slight limitation of physical activity) or functional class III PAH (marked limitation of physical activity). The active ingredient in UPTRAVI®, selexipag, targets the prostacyclin pathway, one of the three main pathways involved in the pathogenesis of PAH. Prostacyclin is a naturally occurring substance that helps regulate blood pressure by binding to receptors in the muscle walls of blood vessels, causing them to relax and widen. Selexipag acts as a prostacyclin receptor agonist, meaning it works similarly to prostacyclin. By binding to prostacyclin receptors, selexipag helps widen the blood vessels, thereby reducing the pressure within them.
Market impact:
UPTRAVI® was originally discovered and synthesized by Nippon Sinyaku Co Ltd, a pharmaceutical company based in Kyoto, Japan. Nippon developed UPTRAVI® in collaboration with Acetelion Pharmaceuticals, which later became part of Johnson & Johnson in an acquisition costing approximately USD 30 billion.
UPTRAVI® is approved for marketing in the United States (approved by the FDA in December 2015) and in Europe (approved by the EMA in May 2016).
UPTRAVI® generated sales of USD 1.817 billion for Johnson & Johnson in 2024. This represented about a 14.9% increase in sales compared to 2023.
Patent protection:
There are patent rights protecting various aspects of UPTRAVI®.
For example, in Europe, EP2447254 relates to a particular crystalline form (polymorph) of selexipag. Claim 1 relates to a Form-I crystal of 2-{4-[N-(5,6-diphenylpyrazin-2-yl)-N-isopropylamino]butyloxy}-N-(methylsulfonyl)acetamide, showing diffraction peaks in its X-ray powder diffraction spectrum at least at the following angles of diffraction 2θ: 9.4 degrees, 9.8 degrees, 17.2 degrees and 19.4 degrees, wherein the X-ray powder diffraction diagram is obtained by using Cu Kα radiation.
Three notices of oppositions were filed to challenge the validity of EP2447254. The Opposition Division rejected these oppositions. The decision of the Opposition Division was subsequently appealed by the second opponent. However, the Board of Appeal considered the claims as granted to meet the requirements of the European Patent Convention and dismissed the appeal.
This patent family includes two divisional filings in Europe, EP3275871 and EP3689855.
Revocation of EP3275871 – T1994/22
EP3275871 was recently revoked by the Board of Appeal in T1994/22. In revoking the patent, the Board of Appeal overturned the Opposition Division’s decision to reject the filed opposition.
Claim 1 of the main request related to a Form-II crystal of 2-{4-[N-(5,6-diphenylpyrazin-2-yl)-N-isopropylamino]butyloxy}-N-(methylsulfonyl)acetamide, showing diffraction peaks in its X-ray powder diffraction spectrum at least at the following angles of diffraction 2θ: 9.0 degrees, 12.9 degrees, 20.7 degrees and 22.6 degrees, wherein the X-ray powder diffraction diagram is obtained by using Cu Kalpha radiation
The Appellant-Opponent argued that claim 1 of the main request lacked an inventive step in view of the patentee’s own expired composition of matter patent, EP1400518A1.
G2/21 – taking into account the effect demonstrated in post-published data
The Respondent-Patentee relied on post-published data and submitted that Form II as claimed had an improved photostability over Form III.
The Appellant-Opponent argued that the application as filed made no mention of photostability of Form II, let alone contained any evidence of it. Based on G2/21, the effect of improved stability could not be relied on.
According to Headnote 2 of G2/21, “a patent applicant or proprietor may rely upon a technical effect for inventive step if the skilled person, having the common general knowledge in mind, and based on the application as originally filed, would derive said effect as being encompassed by the technical teaching and embodied by the same originally disclosed invention”.
Considering Headnote 2 of G2/21, the Board considered the question to be answered in this case was whether the effect relied on by the respondent and demonstrated in post-published experimental data can be derived by the skilled person, having the common general knowledge in mind, and based on the application as filed, as being encompassed by the technical teaching and embodied by the same originally disclosed invention.
The Respondent-Patentee relied on statements made in T116/18 as regards G2/21 and submitted that based on passages of the application as filed, referring to the provision of a novel crystal of selexipag and to a pharmaceutical product of “high quality for which constant effect can always be shown and a form which is hand easily industrially”, the skilled person would have understood that the effect of improved photostability was implied by or at least related to the technical problem initially suggested in application as filed.
In line with T116/18, the Board of Appeal acknowledged that the mere fact that (improved) photostability was not contained in terms of a positive verbal statement in the application as filed and no data in the application as filed was provided, as such, did not imply that the effect could not be relied on. The Board also agreed with T116/18 that the purported technical effect together with the claimed subject-matter need only be conceptually comprised by the broadest technical teaching of the application as filed.
However, the Board found that the paragraphs relied on by the patentee merely related to a sweeping statement regarding high quality and easy industrial handleability, which covered a plethora of potential advantageous properties. The Board stated that if such sweeping statement was sufficient, a reference to high quality would be sufficient to invoke whatever technical effect as being encompassed by an application as filed in the sense of G2/21.
The Board considered the effect of photostability not to be encompassed by the teaching of the application as filed. Even if it did, the Board found the passages relied on by the Respondent-Patentee referred to Forms I to II in equal terms and that they did not teach that Form II is preferred, let alone that it should exhibit a photostability that is improved compared with Form I and Form III.
Consequently, the Board found that the improved photostability of Form II as demonstrated in the post-published data could not be taken into account in the assessment of the technical effects achieved by the distinguishing feature.
Lack of inventive step in view of EP1400518A1
The distinguishing feature between Example 84 of EP1400518A1 and claim 1 of the main request was the crystalline form, namely Form II, of selexipag.
Regarding the technical effect, the Respondent-Patentee relied on five properties, namely: stability, particle size distribution, concentration of residual solvents, level of impurities and improved photostability.
As discussed above, the Board found that improved stability could not be taken into account. The Board also found that Form II exhibited the best stability but only intermediate industrial processibility, intermediate residual solvent content and intermediate amount of residual impurities (compared to the other crystalline forms). In arriving at this view, the Board considered there was no balance of beneficial properties for Form II according to the main request.
The Board found that there was nothing unexpected in finding a polymorph that is optimum for one property but only intermediate for several other properties. The selection of Form II was therefore an arbitrary selection from a host of alternatives covered by the closest prior art.
Claim 1 of the main request was found to lack an inventive step in view of EP1400518A1. Claim 1 of auxiliary requests 1 to 5 were also found to lack an inventive step for the same reasons.
The decision under appeal was set aside and the patent was revoked.
Pending application EP3689855
The pending claims relate to methods of producing Form I and Form II crystals of 2-{4-[N-(5,6-diphenylpyrazin-2-yl)-N-isopropylamino]butyloxy}-N-(methylsulfonyl)acetamide.
Patent support from Secerna
Our team has a wealth of experience gained from working with world leaders in the chemistry and pharmaceutical disciplines encompassing new chemical entities, materials science, agricultural chemistry, chemical processes and formulation technology. We have also worked extensively in the medical device materials and carbon nanotechnology sectors.
For intellectual property advice relating to your next project, please get in touch. Our team will be happy to assist. Contact us here.



