Welcome to “A to Z: Drugs in Highlight
This series is designed to delve into the intricate realm of pharmaceutical drugs. As the world of pharmaceuticals continues to evolve with new discoveries and emerging challenges, we aim to shed light on the diverse array of medications available today, ranging from blockbuster medications to those that may not be as familiar. Whether you’re a healthcare professional, a student, or simply someone with a curious mind, “A to Z: Drugs in Highlight” promises to be an enlightening and engaging series.
T is for TECFIDERA®
TECFIDERA® (from Biogen) is a disease modifying therapy used for treating people with relapsing-remitting Multiple Sclerosis (MS).
How it works:
MS is a disease of the central nervous system where the immune system attacks the protective covering around your nerves (myelin). When the myelin is damaged (demyelination) messages don’t pass along your nerves as efficiently. The areas of damage are called lesions. Symptoms of MS include fatigue, unusual feelings in your skin (such as pins and needles, or numbness), problems with eyesight, and memory issues. There are three types of MS: relapsing-remitting MS (patient has a flare-up of symptoms (relapses) followed by periods of recovery (remissions), secondary progressive MS (stage of MS coming after relapsing remitting MS for many people), and primary progressive MS (from the first (primary) symptoms it is progressive).
TECFIDERA® is used to treat relapsing-remitting MS. The active ingredient in TECFIDERA® is dimethyl fumarate. Its mechanism of action is not fully understood. However, preclinical studies have indicated that dimethyl fumarate activates the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcription pathway. Dimethyl fumarate has been shown to up regulate Nrf2-dependent antioxidant genes in patients. This reduces the inflammation that causes the damage in the brain and spinal cord.
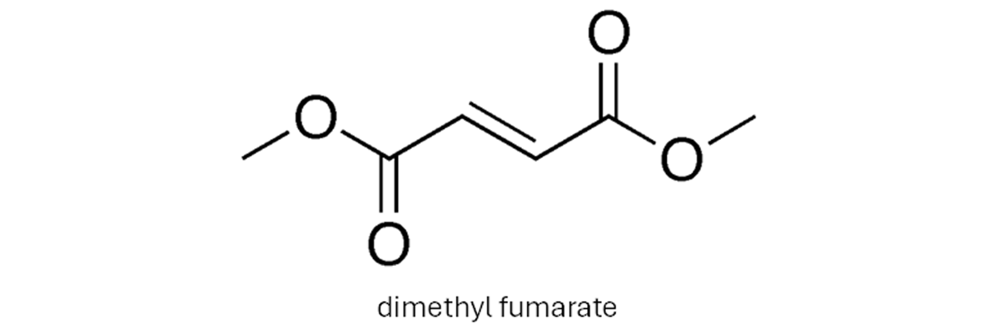
The starting dose for TECFIDERA® is 120 mg twice a day orally. After seven days, the dosage is increased to 240 mg twice a day. In a study, people taking TECFIDERA® saw a greater than 50% drop in the number of relapses compared to a placebo.
Market impact:
TECFIDERA® is approved for marketing in the United States (approved by the FDA in March 2013) and in Europe (approved by the EMA in January 2014).
TECFIDERA® reached its peak sales revenue in 2019, generating approximately USD 4.4 billion. The sales of TECFIDERA® have decreased since 2019 (USD 4.4 billion in 2019 to USD 1.4 billion in 2022) primarily due to the entry of generic versions of the drug into the market.
Patent protection:
There are patent rights protecting various aspects of TECFIDERA®. For example, in Europe, EP2653873 relates to compositions for treating multiple sclerosis. EP2653873 was “divided out” from EP2137537 (EP2137537 was revoked by the Opposition Division – the decision was upheld on appeal by the Board of Appeal (see T1773/16)). Claim 1 of EP2653873 reads:
A pharmaceutical composition for use in treating multiple sclerosis, the composition comprising:
(a) dimethyl fumarate or monomethyl fumarate, and
(b) one or more pharmaceutically acceptable excipients,
wherein the composition is to be administered orally to a subject in need of treatment for multiple sclerosis, and wherein the dose of dimethyl fumarate or monomethyl fumarate to be administered is 480 mg per day.
Claims 5 and 6 of EP2653873 read:
Dimethyl fumarate or monomethyl fumarate for use in treating multiple sclerosis, wherein the dimethyl fumarate or monomethyl fumarate is to be orally administered to a subject in need of treatment for multiple sclerosis at a dose of 480 mg per day.
Dimethyl fumarate or monomethyl fumarate for use as in claim 5, wherein the dimethyl fumarate or monomethyl fumarate is the only neuroprotective compound to be administered.
Fourteen notices of opposition were filed to challenge the validity of EP2653873. The Opponents requested revocation of the patent in its entirety on the basis that (i) the subject-matter of the patent lacked novelty and an inventive step, (ii) the patent was insufficiently disclosed, and (iii) the subject-matter of the patent extended beyond the content of the application as filed and the earlier application as filed (“added subject-matter”).
The Opposition Division found granted claim 6 to add subject-matter. In particular, the Opposition Division found the combination of using dimethyl fumarate (or monomethyl fumarate) as the only neuroprotective compound, and a method of treatment administering orally 480 mg per day of dimethyl fumarate (or monomethyl fumarate) was not directly and unambiguously derivable from the application as filed. The Opposition Division found the claims of auxiliary requests 1, 3, 4, 5, 8, 10, 11, 13, 14, 15 and 18 (which all contain a claim directed to dimethyl fumarate (or monomethyl fumarate) as the only neuroprotective compound to be administered) to also add subject-matter.
Auxiliary request 2 differed from the claims as granted except granted claims 6-8 had been deleted. The Opposition Division found the claims of this request to be insufficiently disclosed. In particular, the Opposition Division found there to be serious doubts about the therapeutic efficacy of dimethyl fumarate on primary progressive MS when used in an amount of 480 mg per day administered orally. The Opposition Division found that auxiliary requests 6, 7 and 9 were also insufficient for the same reasons.
The Opposition Division ultimately maintained the patent in amended form on the basis of the claims of auxiliary request 12. Claim 1 of auxiliary request 12 reads:
A pharmaceutical composition for use in treating relapsing remitting multiple sclerosis (RRMS), the composition comprising:
(a) dimethyl fumarate or monomethyl fumarate, and
(b) one or more pharmaceutically acceptable excipients,
wherein the composition is to be administered orally to a subject in need of treatment for RRMS, and wherein the dose of dimethyl fumarate or monomethyl fumarate to be administered is 480 mg per day.
The Decision of the Opposition Division has been appealed by the patent proprietor (Biogen) as well as many of the Opponents. The deadline for filing their statements of grounds of appeal was 11 April 2025.
Patent support from Secerna
Our team has a wealth of experience gained from working with world leaders in the chemistry and pharmaceutical disciplines encompassing new chemical entities, materials science, agricultural chemistry, chemical processes and formulation technology. We have also worked extensively in the medical device materials and carbon nanotechnology sectors.
For intellectual property advice relating to your next project, please get in touch. Our team will be happy to assist. Contact us here.



