Welcome to “A to Z: Drugs in Highlight
This series is designed to delve into the intricate realm of pharmaceutical drugs. As the world of pharmaceuticals continues to evolve with new discoveries and emerging challenges, we aim to shed light on the diverse array of medications available today, ranging from blockbuster medications to those that may not be as familiar. Whether you’re a healthcare professional, a student, or simply someone with a curious mind, “A to Z: Drugs in Highlight” promises to be an enlightening and engaging series.
S is for STRIBILD®
STRIBILD® (From Gilead Sciences Inc.) is used to treat patients infected with human immunodeficiency virus type 1 (HIV-1), a virus that causes acquired immune deficiency syndrome (AIDS).
How it works:
STRIBILD® is a combination medication containing four active ingredients: elvitegravir, cobicistat, tenofovir disoproxil and emtricitabine.
- Elvitegravir is an integrase inhibitor that blocks the HIV-1 enzyme integrase, which is crucial for the virus’s replication.
- Cobicistat enhances the effect of elvitegravir by extending its duration of action.
- Tenofovir disoproxil is a prodrug that converts into the active substance tenofovir in the body. Both tenofovir and emtricitabine are reverse transcriptase inhibitors, which block the activity of the HIV-1 enzyme reverse transcriptase, preventing the virus from replicating.
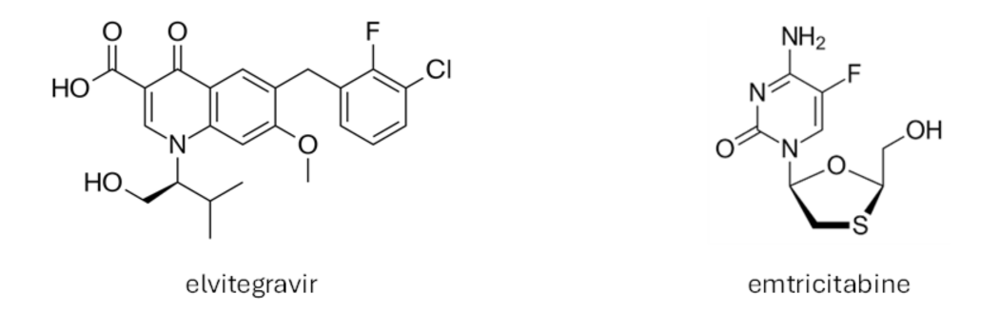
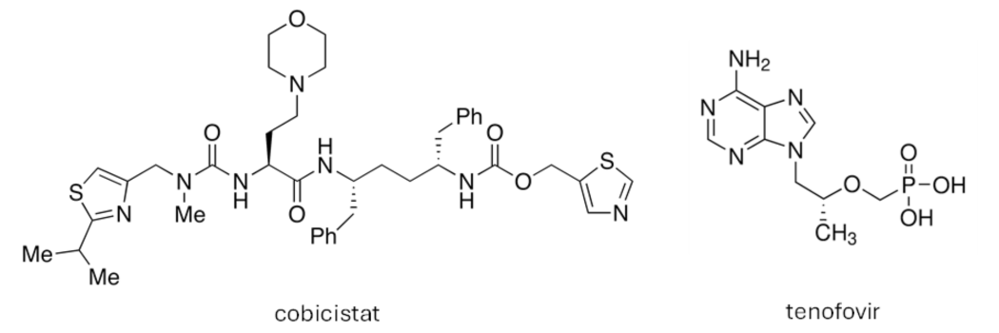
By targeting both integrase and reverse transcriptase, STRIBILD® effectively reduces the amount of HIV-1 in the blood. While STRIBILD® does not cure HIV-1 infection or AIDS, it helps to protect the immune system from damage and delays the onset of infections and diseases associated with AIDS.
Market impact:
STRIBILD® is approved for marketing in numerous countries including in the United States (approved by the FDA in August 2012) and Europe (approved by the EMA in May 2013).
STRIBILD® was one of the earlier single-tablet regimens for HIV, combining multiple antiretroviral drugs into one pill. This convenience helped it gain a strong market position initially. However, over time, newer therapies like Gilead’s Biktarvy and two-drug regimens have become more popular, leading to a decline in STRIBILD®’s market share.
Patent protection:
There are patent rights protecting various aspects of the STRIBILD® technology in the United States and Europe. For example, in Europe:
EP1564210 and EP1636190 provide protection for elvitegravir. In particular, EP1564210 relates to the use of a 4-oxoquinoline compound represented by the following formula [I] of a pharmaceutically acceptable salt thereof:

wherein Cy, X, Y, R1, R2 and R31 are broadly defined, for the manufacture of a medicament for treating HIV infectious disease. Elvitegravir is specifically protected in claim 26. Although the European patent expired in 2023, there remains supplementary protection certificates (with paediatric extension) in force across Europe based on EP1564210 which extend the term of protection for elvitegravir until 26 November 2028. See, for example, SPC/GB13/065.
EP2118082 relates to a long list of compounds for modifying the pharmacokinetics of a co-administered drug. Cobicistat is included amongst the listed compounds. A pharmaceutical composition comprising the combination of cobicistat, elvitegravir, emtricitabine and tenofovir is also envisaged in claim 6.
Patent support from Secerna
Our team has a wealth of experience gained from working with world leaders in the chemistry and pharmaceutical disciplines encompassing new chemical entities, materials science, agricultural chemistry, chemical processes and formulation technology. We have also worked extensively in the medical device materials and carbon nanotechnology sectors.
For intellectual property advice relating to your next project, please get in touch. Our team will be happy to assist. Contact us here.



